The Regular Lines of Antigen-Antibody
Interactions in vitro
Antibodies
are Bound Irreversibly to Their Antigenic Determinants Under Physiological Conditions
t
An
analytical review
Viggo Bitsch
viggo.bitsch@gmail.com
Published Oct. 26, 2017
ISBN 978-87-994685-2-2
©
2017 Viggo Bitsch
Abstract
Viggo Bitsch: The regular lines of antigen-antibody interactions in vitro. Antibodies are bound irreversibly to their antigenic determinants under physiological conditions.
This analytical review aims to clarify antigen-antibody interactions in vitro. Aspects of the antigen-antibody binding reactions are evaluated, and important conclusions are made. All are summarized in Section 3 at the end of each subsection. Results related to the performance of sensitive and standardized tests demonstrating antigens and antibodies are overviewed in a final section. Only the most important relations will be mentioned in this abstract.
Antibodies are bound firmly and irreversibly to their antigenic determinants on a virus under physiological conditions. The binding reaction does not lead to an equilibrium state. The sensitivity of antigen-antibody tests will consequently be variable and adjustable.
Neutralization of virus by antibody in a virus-antibody mixture and in the dilutions of a neutralization test proceeds as two separate reactions, i.e., 1) a first-order reaction caused by neutralizing antibodies being bound monovalently to their antigenic neutralization determinant on virions and 2) a supplementary “over-neutralization” reaction that can be attributed to the formation of virus aggregates by the di- and polyvalent antibodies which predominantly are non-neutralizing. The aggregation reaction is prompt and short-lasting. In contrast, the first-order neutralization reaction is slowly progressing but enduring, and the sensitivity of an antibody neutralization test is temperature-dependent and basically directly proportional to the reaction time.
The neutralizing effect of antibodies by aggregating virions is enhanced by complement. The binding of an antibody to its antigenic determinant will sensitize that antibody for binding to the polyvalent complement component C1q, after which the virus will be inactivated by the inclusion of the virus-antibody complex in virus-antibody aggregates. If complement in optimal amounts is added to mixtures of virus and specific antibody in the dilutions of neutralization test, two different aggregation reaction rates will be observed. Practically instantly, the complement will aggregate all pre-formed virus-antibody complexes, after which the reaction will be of first-order, following the first-order binding of non-neutralizing antibodies to their antigenic determinants.
The huge virus-neutralizing potential of the non-neutralizing antibodies, especially the acute-infection-phase IgM antibodies, in a joint action with complement indicates the fundamental role of these antibodies in preventing and combatting infectious diseases.
The antigen-antibody interaction formula expresses the regular lines of antigen-antibody interactions not comprising aggregation. Sensitive and specific antigen-antibody tests elaborated based on the relationships in this formula have been used for a long time in the veterinary medical field.
1. Introduction.
This analytical review clarifies and documents the regular lines of antigen-antibody interactions in vitro.
An antibody neutralizing a virion by attachment to its antigenic determinant is a neutralizing antibody, and the corresponding epitope on the virion will be termed the antigenic neutralization determinant. Antibodies not neutralizing a virion by being bound to their antigenic determinant on the virus are non-neutralizing antibodies.
The lines of antigen-antibody interactions are significant for elaborating adequate tests for demonstrating antigens or antibodies. These tests are usually performed so that test material is allowed to react appropriately with a specific reactant, antigen, or antibody, after which a detection system is used to confirm if, or to which extent, a reaction has taken place. Reaction conditions used in such tests have commonly been chosen empirically, rather than from knowledge of regular lines of antigen-antibody reactions.
In
the following, using a herpesvirus model, the influence of the
various variables on neutralization in a virus-antibody mixture and
in
the serial dilutions of neutralization tests
is analyzed, and the implications of the relationships demonstrated
for the elaboration of appropriately sensitive antigen-antibody tests
are evaluated. Figures 1-6 and 7-8 are
from Bitsch
1978
[1] and Bitsch
and Eskildsen
1982
[2], while Figure 9
shows unpublished data.
2. The regular virus-antibody neutralization reactions in vitro.
2.1. The regular neutralization reactions without interaction by complement.
In very early in vitro investigations performed with bacteriophages and animal viruses, the neutralization rate in a virus-antibody mixture was found to be logarithmically linear with the reaction time (Andrewes and Elford 1933) [3], proportional to the antibody concentration (Burnet et al. 1937) [4], temperature-dependent (Dlbecco et al. 1956) [5], and independent of the virus concentration (the Percentage Law) [3]. Despite these findings, it became generally acknowledged that the reaction in a conventional neutralization test would lead to a state of equilibrium in compliance with the Law of Mass Action, most likely because no substantial progression of the reaction was observed in a neutralization test over the first couple of hours.
The 1978 study [1] became significant for understanding the neutralization reactions, being the only investigation of antigen-antibody interactions comprising the short- and long-term influence of all relevant variables, i.e., the concentrations of the reacting antigens and antibodies, and the reaction temperature and time.
Figures 1, 2, and 3 are from the 1978 study [1]. Figure 1 shows the semi-logarithmic progression of neutralization in a mixture of virus and an appropriate amount of antibody, which is linear following Eq. 1 below. The initial virus concentration corresponds to the dose used ordinarily in a conventional neutralization test (100 TCID50 per inoculation dose). In Figure 6b, an identical linear progression is recorded with a much higher virus dose with serum diluted 1:4. In Figure 2 the kinetics of neutralization in neutralization tests at 37 oC with very short reaction periods and a traditional low virus dose, while Figure 3 shows the log-log kinetics in tests also at 37 oC for reaction periods up to 24 hours with varying virus concentrations. Here, with reaction periods from approx. 2-3 hours onwards, the log-log neutralization lines for all virus concentrations show a slope coefficient of 1, demonstrating a first-order progression of neutralization, whereas with shorter reaction periods a supplementary “over-neutralization” phenomenon is observed. Over-neutralization could not be explained but was concluded to be a regular condition.
Figure 1. Kinetics of virus neutralization in two virus-antibody mixtures with different low antibody concentrations. From Bitsch 1978 [1]
Virus: BoHV1. When plotted semi-logarithmically, the progression of virus neutralization is linear with the neutralization rate being 4 times higher for the stronger antibody mixture.
Figure 2. Kinetics of neutralization for 3 sera in neutralization tests with very short
reaction periods for the virus-serum mixtures. From Bitsch 1978 [1].
Virus: BoHV-1. VNA: virus-neutralizing antibody. Preincubation: reaction time for virus-serum mixtures before inoculation of cultures. The virus dose was approx. 100 TCID50. Serum was late-infection samples after nasal infection (IgG). After incubation of virus-serum mixtures at 37 oC for the indicated periods, 4 tissue culture roller tubes containing maintenance medium were inoculated with virus-serum mixtures from each serum dilution, involving that the mixtures were diluted 1:10 immediately on inoculation. Serum B3 was tested in parallel by inoculation of cultures without medium but with the addition of the medium after 3 hours. Results were plotted logarithmically. The dotted line shows the results from testing Serum B3 on cultures without medium.
Figure 3. Kinetics
of neutralization in neutralization tests at 37 oC
with relatively long periods of virus-serum incubation and varying
virus concentrations. From
Bitsch 1978 [1].
Virus and definitions: see Fig.
2. Results were plotted logarithmically. The neutralization lines are
seen to be identical, although varying with the virus
concentrations. From approx. 2-3 hours onwards, they can be
considered to be linear with a slope coefficient of 1. The reaction lines can be seen as a continuation of the lines in Fig. 2.
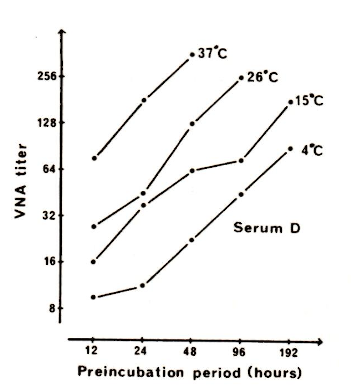
The linear progression of virus inactivation in a neutralization test with a log-log slope coefficient of 1 is also seen in Figure 4 with the reaction temperature of 4 oC for up to 8 days of reaction, and in Figure 5 at 37, 26, 15, and 4 oC for up to 2, 4, 8, and 8 days, respectively.
Figure
4. Kinetics
of neutralization in a cv/v neutralization test at low temperature (4
oC)
with varying virus doses and long-term incubation periods for
virus-serum mixtures from 12
to 192 hours. From Bitsch
1978 [1] .
Virus
and definitions: see Fig.
2.
The log-log neutralization lines show the same slope coefficient of
1.
Figure 5. The influence of the reaction temperature on the progression of neutralization in
neutralization tests. From Bitsch 1978 [1].
The virus dose was approx. 1000 TCID50 and incubation of virus-serum mixtures were made at 37,
26, 15, and 4 oC from 12 to 48, 96, 192, and 192 hours, respectively. The log-log neutralization
lines were all linear with a slope coefficient of 1, and a semi-logarithmic direct proportionality was
found over the temperature range between the temperature and antibody titer.
In Figures 3, 4, and 5, the log-log progression of neutralization is recorded. All reaction lines show a slope coefficient of 1 from approx. 2-3 hours onwards at 37 oC, and at least from 12 hours onwards at 15 and 26 oC, and from 24 hours onwards at 4 oC. A log-log slope coefficient of 1 indicates a first-order reaction (from log y = log x + log a, follows y=ax). Fig. 5 demonstrates a semi-logarithmically linear relationship between the antibody titer and reaction temperature.
It is
unquestionable that the inactivation of the virus in a conventional
neutralization test results from two completely different reactions. With
extended reaction times, the neutralization proceeds exclusively as a slowly
progressing, enduring reaction of first order, while a prompt and short-lasting
“over-neutralization” dominates with short
reaction periods.
The neutralizing capacity of an antibody medium containing specific
antibodies to a virus can theoretically be measured in two ways: either by
determining 1) the neutralization rate factor or 2) the virus-neutralizing
antibody titer in a conventional test, in both cases with the use of a selected
virus concentration. The formulae for 1) the neutralization rate (r) and 2) the
neutralization rate factor (k) are as
follows:
where V0 and VT are titers of the infective virus after 0 and T hours of reaction and D is the dilution factor of the antibody medium where the virus dose has been reduced to 1 TCID50 after T hours of reaction. The dilutions are checked for infectivity by inoculation onto tissue cultures.
Addendum: A residual fraction of virus neutralized at a lower rate or a persistent fraction.
When
one particular serum was tested in the same way as Serum A (Fig.
3),
a somewhat irregular course of neutralization was observed, as the
highest virus dose
after long reaction periods in mixtures with low antibody
concentrations gave reduced neutralization (Figs.6a
and 6b).
The rates of neutralization for this virus dose by the serum in
dilutions 1:4 and 1:16 demonstrated that the deviation was associated
with a
residual fraction of the virus being neutralized at a lower rate.
Figures 6a (left) and 6b (right). Irregular progression of neutralization for a certain serum sample in a cv/va neutralization test with high virus doses and long incubation periods. From Bitsch 1978 [1].
Virus and definitions: see Figure 2. Figure 6a shows that the neutralization curve for the serum, when tested with the highest virus dose and incubation for 6 to 24 hours, drops out. Furthermore, Figure 6b confirms that the high virus dose is linearly neutralized in dilution 1:4 within 2 hours, while in dilution 1:16 a residual fraction is neutralized at a lower rate.
2.2. The supplementary complement-dependent neutralization reaction.
The investigations were performed with Suid herpesvirus 1 (SuHV-1) with extended reactions at 37 oC [2]. First, the effect of complement on the progression of virus inactivation in a neutralization test was investigated (Figure 7), using serum from a pig 13 days after nasal infection containing predominantly specific IgM and a low level of IgG antibodies, cf. Figure 8. The optimal effect of complement was not obtained if added at the start of the virus-serum incubation (neutralization line 1-1). The extended reaction periods gave neutralization lines with a log-log slope coefficient of 1, indicating a first-order reaction with increasing reaction times.
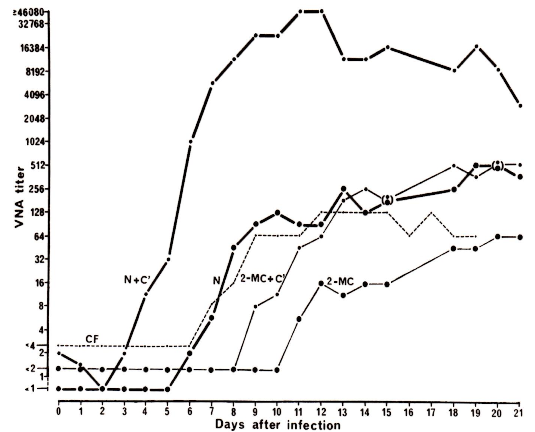
Figure 7. The effect of complement on the progression of neutralization in a convalescent-phase serum. From Bitsch and Eskildsen 1982 [2]
Virus: SuHV-1. Serum was taken 13 days after nasal infection. Virus-serum mixtures were incubated at 37 oC, and titers were recorded by inoculation of cultures after 3, 6, 12, and 24 hours. K0 and K1: no complement (K0) or heat-inactivated complement (K1) was added at the start of virus-serum incubation. For the reactions 1-1, 2-2, 3-3, and 4 complement was added at the start of incubation and after 5, 11, and 23 hours, respectively.
Figure 8 shows results obtained in neutralization tests with serum from a nasally infected pig during the first 21 days after infection. Tests were performed with reaction at 37 oC for 24 hours. Sera were treated with 2-mercaptoethanol to inactivate IgM antibodies, and both treated and non-treated samples were tested with and without the addition of complement. Regarding the virus-inactivation titers of the non-neutralizing and neutralizing IgM and IgG antibodies visualized by the action of complement, see the legend in Figure 8. Titers are determined by the reacting antibodies in the highest concentrations, which in conventional tests will be neutralizing and in complement-enriched tests will be non-neutralizing antibodies.
Figure 8. Effect of complement on reactions in conventional and complement-enriched neutralization tests for sera collected from an animal during the first 21 days after nasal infection. From Bitsch and Eskildsen 1982 [2].
Virus:
SuHV-1. The sera were inactivated at 56 oC
for 30 min. and tested, either untreated (N) or treated with
2-mercaptoethanol (2-MC), which will inhibit the neutralizing effect
of IgM antibodies, but leave IgG antibodies unchanged. In both cases,
they were tested with and without the addition of complement (C´). The
virus-serum mixtures were incubated at 37 oC
for 24 hours, and where used complement was added after 23 hours of
reaction. Results from a complement fixation test are also shown
(CF).
Titers
measured for antibodies are indicated by the symbols as follows:
N+C': non-neutralizing IgM antibodies;
N: neutralizing IgM
antibodies;
2-MC +C': non-neutralizing IgG antibodies;
2-MC:
neutralizing IgG antibodies.
2.3. Major findings regarding the lines of virus neutralization in vitro.
Viruses are neutralized under natural conditions in neutralization tests by antibodies in three different ways following specific reaction lines. These reactions are 1) the first-order neutralization reaction, 2] the “over-neutralization” reaction, and 3) the supplementary complement-dependent neutralization [1,2].
The first reaction is seen in a conventional test in the absence of complement with extended reaction periods. This first-order neutralization is slowly progressing and enduring with increasing reaction periods. The titer and the test sensitivity achieved are temperature-dependent and proportional to the reaction period. The reacting antibodies are neutralizing.
The second reaction "over-neutralization" is the early prompt neutralization in a conventional neutralization test without interference by complement. The reaction is rapid and short-lasting and highly dependent on the antibody concentration. The reacting antibodies are predominantly non-neutralizing. This over-neutralization reaction due to simple virus aggregation will not be seen in a complement-enriched neutralization test, see below.
The third supplementary complement-dependent neutralization reaction is caused specifically by non-neutralizing antibodies in a joint action with the complement component C1q. The reaction occurs under controlled conditions in a complement-enriched neutralization test. It is prompt immediately after complement addition because of the immediate binding to the virus-antibody complexes already formed. However, with increasing reaction times, it proceeds as a reaction of first order, following the continuous first-order binding of non-neutralizing antibodies to their antigenic determinants.
3. Discussion and conclusions on specific items.
3.1. The over-neutralization reaction in a conventional neutralization test.
In
the 1978 study [1], over-neutralization
was defined
as the early and regular neutralization reaction in virus-antibody
mixtures of neutralization test, being more advanced than expected
from a linear progression of neutralization following Eq.2 below.
Over-neutralization is rapid and short-lasting and was not seen in the dilutions of herpesvirus neutralization tests with reaction periods exceeding
2-3 hours at 37 oC,
implying that it will not appear in mixtures with low antibody
concentrations.
In 1983, Brioen et al. [14] reported that aggregation of virions by di- and polyvalent antibodies resulted in the inactivation of the virus (see also Thomas et al. 1985 [15], Thomas et al. 1986 [16], and reviews by Klasse and Sattentau 2001 [17], and Reading and Dimmock 2007 [6]). Their report described a new method of virus inactivation by antibodies.
This second neutralization method immediately explained the over-neutralization reaction as an early virus aggregation response by the di- and polyvalent antibodies, predominantly the non-neutralizing ones. One reason for this speedy
reaction is that the many different antibodies aggregate
synergistically.
The second reaction identified in the 1978 study was the slowly
progressing, first-order inactivation by neutralizing antibodies binding monovalently to their antigenic neutralization
determinant. In the serial dilutions of a conventional neutralization test, the concentration of aggregating antibodies will soon be diminished to the extent that the continuing reaction by neutralizing antibodies will be exclusively monovalent and of first order.
An aggregation reaction was not seen in earlier measurements of neutralization rates [3,4]. A likely explanation is that the antibody samples used had high antibody concentrations, because of which the aggregation reaction was diluted away in the dilutions used in the experiments.
Conclusions
summarized.
· 1. Over-neutralization was in the 1978 study defined as
neutralization exceeding what should
be expected from the first-order
neutralization reaction and was concluded to be a regular
feature of a
neutralization reaction.
2. The reaction can be attributed to a particular ability
of antibodies, due to their di- or poly-
valency, to aggregate virions.
3. Over-neutralization
is seen only with moderate to high antibody concentrations. In a
neutralization test, it will be negligible in dilutions with antibody
concentrations below a
certain level.
4. Over-neutralization can readily be diluted away.
5. Over-neutralization will be negligible in
neutralization tests with reaction periods beyond
a given length.
6. Conventional
neutralization tests should be performed with reaction conditions eliminating
interference
by aggregation.
3.2. The first-order progression of neutralization in a conventional neutralization test.
When evaluating relationships between variables of a neutralization test it is important to keep in mind that the neutralizing potency or antibody titer of a sample is recorded by the dilution factor (D) of that particular dilution of the antibody medium, where the virus dose (V0) after a reaction period of T is neutralized to 1 TCID50 (VT will be 1). DT will be the titer obtained after T hours of reaction, implying that in the dilution 1/D of the antibody medium VT will be 1, and log VT will be 0. The binding of neutralizing antibodies to their antigenic neutralization determinant on a virion will abolish its ability to adhere to and infect susceptible cells. Neutralization of a virus in vivo may be more complicated (cf. reviews by Reading and Dimmock (2007) [6] and Klasse (2014) [7]), but in the present evaluation of neutralization reactions in vitro, we will stick to this simple concept.
Considering the linear progression of neutralization in the dilution series of a conventional neutralization test with extended reaction periods as shown in Fig. 3, it will be understood that the virus-neutralizing antibody titer recorded is proportional to the reaction time. A titer is recorded by the dilution factor D for that particular dilution where the virus dose is neutralized to 1 TCID50 after T hours of reaction (cf. Figs. 1 and 6b). If D1 and DT are the titers obtained in a neutralization test after 1 and T hours of reaction, this relationship can be expressed as follows:
(Eq. 3) DT = D1T (or log2DT = log2D1 + log2T).
In Figs. 3 to 7, the log-log plots of the relationship between antibody titers and extended reaction periods are straight lines with a slope of 1, confirming that the neutralization is proceeding as a first-order reaction in full compliance with Eq. 2.
The
results shown in Fig. 5 demonstrate the semi-logarithmic linear
relationship between the progression of neutralization and the reaction
temperature as documented by Dulbecco et al. (1956)[5]. A
decrease in temperature by 10 oC within the interval examined will
reduce the test sensitivity approximately by a factor of 2.3, and the antibody
titer level or test sensitivity obtained at 37 oC after 24 hours of reaction will not be achieved in
tests at room temperature or 4 oC until after 4 and 16 days,
respectively. This underlines that the reaction temperature in sensitive
antigen-antibody tests should not be below 37 oC.
· 1. The antibody reaction in a conventional neutralization
test with extended reaction is enduring
and of first order.
2. The sensitivity of an antibody
test following a first-order antibody reaction is proportional to
the reaction
time.
3. The sensitivity of a
first-order neutralization test is temperature-dependent to such an extent
that
37 oC should be the standard reaction temperature.
4. A standard neutralization test
should be performed with a reaction at 37 oC for 24 hours.
3.3. The effect of complement on neutralization.
Yoshino and Taniguchi (1964) [8], see also Yoshino and Morishima (1971) [9], observed an enhanced neutralizing effect in vitro of convalescent-phase antibodies to herpes simplex virus by the addition of complement. Complement-dependent neutralization by antibodies was found also for other viruses. IgM and IgG antibodies are the significant immunoglobulins involved in neutralization, being deca- and divalent, respectively, cf. reviews by Oldstone (1975) [10], Cooper and Nemerow (1986) [11], and Goldsby et al. (2000) [12]. The complement component C1q is equipped with six globular heads capable of binding strongly to the Fc region of antibody molecules, but not unless the antibody has sensitized by being coupled with its antigenic determinant. As soon as antibody-virus complexes have been caught by complement, they will be included in non-infectious aggregates.
For samples taken shortly after infection (Figs. 7 and 8), the effect of C1q on IgM antibodies was huge. A maximal titer increase of 8-9 base-2 log units was obtained 9-11 days after infection. But also for IgG antibodies a considerable effect, a titer increase of 3 base-2 log units, could be noted. Consistently, an investigation of 24 naturally infected animals a long time after natural infection had shown a mean titer improvement of 3 base-2 log units (Bitsch and Eskildsen (1976) [13].
The effect of complement was reduced if added to the virus-antibody mixtures at the start of the reaction. An optimal effect can be achieved if added even a short time, e.g. 15 min., before inoculation of tissue cultures. The effect of complement was exactly the same when added after 5, 11, or 23 hours of antigen-antibody reaction (Bitsch and Eskildsen (1982) [2].
The neutralization results with extended reaction after the addition of C1q shown in Fig.7 (1-1, 2-2, 3-3, and 4), illustrate the supplementary neutralization effect by complement. The reaction immediately after the addition of complement is practically instant, but thereafter, the neutralization reaction with non-neutralizing antibodies continues as a linear reaction with a slope coefficient of 1, reflecting the continuing first-order binding reaction between the non-neutralizing antibodies and their antigenic determinants.
The first-order binding of non-neutralizing antibodies to their antigenic determinants, disclosed by the complement reaction, is the sum of the first-order binding reactions for the various non-neutralizing antibodies.
The virus inactivation potency by the early polyvalent,
non-neutralizing IgM antibodies as seen in Figure 8 is huge.
Conclusions
summarized.
· 1. The complement component C1q is hexavalent and
will bind to the Fc region of antibodies
sensitized by being bound to their
antigenic determinant on a virion, Because of the polyvalency
of C1q, antibody-virus complexes will thereafter be
included in non-infectious aggregates.
2. The supplementary neutralizing effect of
the complement component C1q is caused by a reaction
with non-neutralizing
antibodies.
3. In a complement-enriched neutralization test, the complement effect is biphasic. After
the addition,
it will react immediately with pre-formed antibody-virus
complexes but thereafter, with
increasing reaction time, it will continue as a reaction
of first order, following the first-order
binding of non-neutralizing
antibodies to their antigenic determinants.
4. The first-order binding of
non-neutralizing antibodies to their antigenic determinants disclosed by
the
complement reaction is the sum of the first-order binding reactions for the various
non-
neutralizing antibodies.
5. With herpesviruses, complement raised IgG
antibody titers by a factor of 8, but for IgM antibodies,
the improvement was immense.
6. A standard complement-enriched
neutralization test should be performed with a reaction at 37 oC
for
24 hours. Complement should be added late to achieve optimal sensitivity.
3.4. The factor q is a temperature-dependent co-determiner of the regular virus-antibody neutralization rate.
Considering the linear progression of neutralization
with reaction periods exceeding 3 hours as seen in Fig. 3, it
will be seen that 1) for any given reaction period, a certain log
increase in virus titer will require a log increase in antibody titer
of a certain size to have this higher virus dose neutralized to 1
TCID50, and 2) for any antibody concentration this same
log increase in virus titer will require a
log increase in
reaction period of exactly that same size to neutralize the virus
dose to 1 TCID50. This is a consequence of the fact that
the slope coefficient of the neutralization lines is 1. This
particular log increase per 1 base-10 log unit of virus, a log
antibody/log virus ratio, is a characteristic of the first-order
neutralization reaction and was in the 1978 study designated the
factor q or the log antibody/log virus equivalence factor of
neutralization [1].
The relations above 1) between antibody and virus
titers, when the reaction time is the same and 2) virus dose and
reaction period, when antibody titers are the same, can be expressed
by the equations:
(Eq. 4) or
, and
where D expresses the antibody titer, lim D is the limit of D as V approaches 1, and V1 is the value of V corresponding to T=1 (the factor q is independent of the log base used in the equations).
By comparing the results in Figure 3 with those in Figure 4 it will be seen that the factor q is dependent on the temperature, being approx. 0.24 at 4 oC and 0.15 at 37 oC. This reduced effect of antibodies at lower temperatures may be explained simply by a reduced number of hits between the reactants due to reduced molecular movements.
Conclusions summarized.
1. The concentration or titer of both the antigen and reacting antibody determines the neutralization
rate in virus-antibody mixtures.
2.
The
antibody-virus equivalence factor of neutralization or the factor q
(a particular log
antibody/log virus ratio) is a central
characteristic of the first-order antigen-antibody
reaction.
3. This factor is dependent on the reaction
temperature.
3.5. On dissociability of antigen-antibody complexes and the Law of Mass Action.
The stability of the binding between antigens and antibodies has been debated since the beginning of interaction studies. The forces connecting the reactants have been hypothesized to be weak and caused by electrostatic forces, hydrogen bonds, van der Waals forces, and hydrophobic forces, cf. Male et al. 2012 [21], Murphy 2012 [22]. These perceptions appear questionable because they do not explain the mechanisms of specificity, i.e., the specific attraction and binding between the various antibodies and their particular antigenic determinants.
In immunology textbooks (Goldsby et al. 2000 [12], Male et al. 2012 [21], Murphy 2012 [22]) and review articles (Svehag (1968) [23], Parren and Burton (2001) [24], Klasse and Sattentau (2002) [17], Reading and Dimmock (2007) [6], Klasse 2014 [7] ) it is generally stated that the antigen-antibody reaction follows, at least principally, the Law of Mass Action (the Law of Chemical Equilibrium) according to which the reaction will lead to an equilibrium state. Conflicting results have in literature been explained in different ways, for example by varying affinity of antibodies. Some authors demonstrated a somewhat higher sensitivity by increasing the reaction time and concluded that bindings might become more stable over time, e.g., Gard 1955 [32], 1957) [33], Svehag 1963 [34], Svehag and Mandel 1964 [35].
When the progression of neutralization in a virus-antibody mixture is measured in probes performed at regular intervals, a semi-logarithmically linear relationship is found [3], cf. Figures 1 and 6b, and Eq. 1. The dilution procedure used in such cases to disclose the actual advancement of neutralization in the samples can be taken to indicate that the neutralization observed is caused by the formation of antigen-antibody complexes that are undissociable under physiological conditions. A steady, linear progression of a monovalent reaction is the maximal rate and significant dissociation should give a diminishing rate.
In the 1978 study, the progression of neutralization in a cv/va test was investigated at varying temperatures for reaction periods up to 48 hours at 37 oC and 8 days at 4 oC (Figures. 3 and 4). After an initial phase characterized by over-neutralization due to the aggregation of virus particles created mainly by non-neutralizing antibodies, the reaction followed the linear relationship seen in individual antigen-antibody mixtures (Eqs. 1 and 2). The 1978 investigations were performed with samples collected long after infection and allowed one definite conclusion: the complexes formed between IgG-neutralizing antibodies and the virus are stable and do not dissociate under physiological conditions. Even after extremely long reaction periods, the neutralization rate is constant. The binding of the non-neutralizing IgM antibodies to their antigenic determinants with extended reaction is also a first-order reaction as shown in complement-enriched neutralization tests (Figure 7) [2].
The neutralization process in vitro of the non-enveloped poliovirus and the enveloped herpesviruses directed by antibodies has been extensively studied, and results for these viruses appear to be similar. This can not be surprising, since significant antigenic neutralization determinants seem predominantly to be glycoproteins and immunoglobulins are basically identical, varying only with specificity. Mandel (1961) [25] found no reversibility of neutralized poliovirus-antibody complexes after reaction at 37 oC for 2 hours. Jerne and Avegno (1956) [26] stated that only one report (Andrewes and Elford (1933) [27] out of 14 cited indicated reactivation of neutralized phages. Andrews and Elford had found for one serum, but not for others, that if a diluted virus-antibody mixture was allowed to stand for some hours before being tested for infectivity, the number of infective particles would be higher than when tested immediately on dilution. Jerne and Avegno found differences among sera collected early and late after immunization, as a late sample appeared to neutralize phages irreversibly, whereas neutralization by serum taken 8 days after the first immunizing injection appeared to be reversible on dilution. It is obvious that the effect of the early serum was related to IgM antibody, but, unfortunately, serum was used undiluted and unheated to activate complement, so the importance of their observation is uncertain.
In investigations of dissociability, no attention was paid to the possibility that the neutralization process might not be mono-factorial. It must be concluded that antigen-antibody complexes as seen with herpesviruses are generally stable under ordinary physiologic conditions. No results contradicting that could be found. Both neutralizing and non-neutralizing IgG and IgM antibodies can be concluded to be firmly bound to their antigenic determinants.
Conclusions summarized.
1. The widely acknowledged concept that
neutralization follows the lines of the Law of Mass
Action, leading to an equilibrium state is not substantiated by results in the literature.
2. No evidence of reversibility of virus-antibody
bindings under physiologic conditions could
be found
either.
3. The specific binding mechanisms for antigenic determinants and their binding sites on
antibodies are unexplained.
4. A
first-order antigen-antibody reaction at extended reaction periods in
a neutralization
test correlates with monovalent binding
and proceeds at a constant rate. Significant
dissociation
should give a diminishing rate.
5. Both neutralizing and non-neutralizing
herpesvirus antibodies are bound firmly to their
antigenic determinants, following the
lines of the antigen-antibody interaction formula
with
extended reaction periods.
3.6. The Percentage Law.
Andrewes and Elford 1933 [3] hypothesized the so-called Percentage Law, implying that the neutralization rate in a virus-antibody mixture is independent of the virus concentration. Burnet et al. 1937 [4] and Dulbecco et al. 1956 [5] accepted their conclusion, which later became widely recognized, although neither documented nor logically explained.
As shown above, the first-order progression of neutralization found in a neutralization test with reaction periods long enough to make the over-neutralization reaction negligible, is in agreement with Eq. 2. However, when this regular reaction was investigated with varying virus doses (Figure 3), the neutralization rate was found dependent on the virus concentration. This appears from Eq. 6, which follows directly from Eqs. 3, 4, and 5. In Eq. 6, the neutralization rate is shown to depend on the virus concentration to a degree further dependent on the antibody-antigen equivalence factor of neutralization (the factor q).
In consequence of the demonstrated invalidity of the Percentage Law, the neutralization rate factor given in Eq.2 is not a constant characteristic of the concentration of the reacting antibody (or titer) as usually claimed, but a variable factor further depending on the virus concentration to a degree determined by the factor q. Consequently. the term neutralization rate factor is preferable to the term neutralization rate constant.
Conclusions summarized.
1. The rate of neutralization in a virus-antibody
mixture depends on the concentration of
antigen as well as the concentration of
antibody and the reaction temperature.
2. The hypothetical percentage law is invalid.
3. The term neutralization rate factor should be
preferred to neutralization rate constant.
3.7. A residual fraction of virus neutralized at a lower rate or a persistent fraction.
Andrewes and Elford 1933 [3] observed in their investigations on neutralization that a fraction of the virus was refractory to neutralization. Burnet et al. 1937 suggested that it was due to an equilibrium state of neutralization, Dulbecco et al. 1956 objected to that, as the fraction was not proportional to the antibody concentration. More theories have been presented to explain the phenomenon, among these that it is caused by the interaction of non-neutralizing antibodies leading to a steric hindrance for neutralizing antibodies (Ashe and Notkins 1966 [20], Massey and Schochetman 1981 [28], see also reviews by Parren and Burton 2001 [24], Klasse and Sattentau 2002 [17], and Reading and Dimmock 2007 [6]).
The 1978 study [1] demonstrated that neutralization in a neutralization test with extended reaction proceeds as a first-order reaction. Initially, however, the reaction was complicated by a rapid and short-lasting aggregation reaction referred to as the "over-neutralization" phenomenon. Both reactions were concluded to be regular. One serum sample, however, showed a deviation with the highest virus dose and long reaction periods, as a residual fraction of the virus was neutralized at a lower rate (Figures 6a and 6b). Several other sera had been examined in pilot studies, but only this serum showed that complication.
The question was how this serum deviated from the others. It had been selected because it originated from a bull at an artificial insemination center, where it had become naturally infected several years before the blood collection. As that animal was a champion bull, his semen was allowed for routine inseminations after the infection had been diagnosed, but preconditioned virological examination with a negative result of the liquid from a preputial washing performed immediately before the semen collection. The virus was demonstrated later occasionally, and at one time it was decided to clarify if a booster injection of culture virus could reduce the risk of re-shedding the virus. So, although the serum originated from a naturally infected animal, it was actually from an experimental animal having been further immunized by the injection of the virus.
The reduced neutralization observed in the 1978 study in antibody dilutions can be explained by the action of non-neutralizing antibodies. Virions will literally in virus-antibody mixtures be coated with antibodies on their surface. This means that the virus particles to be neutralized very late in the course of the process already before the neutralization takes place, or should take place, will have been coated with a variety of non-neutralizing antibodies, which thereby to a degree depending on the reaction time will block for the binding of neutralizing antibodies to their epitopes. The particular circumstances related to the problem observed for this bull point to the possibility that the parenteral immunization had significantly changed the relative concentrations of non-neutralizing and neutralizing antibodies from what is seen in natural antibody media. The antibody samples used by authors reporting a reduced neutralization rate or a persistent fraction of virus had regularly been artificial hyper-immune sera, cf. Andrewes and Elford 1933 [3], Burnet et al. 1937 [4], Dulbecco et al. 1956 [5], and Icenogle et al. 1983 [29], had in fact regularly been artificial hyper-immune sera.
A persistent fraction of the virus, or a residual fraction being neutralized at a lower rate, may consequently be considered an artifact.
Conclusions summarized.
1. Reduced neutralization - a persistent fraction
of the virus - is a complication, which has been
seen in experiments with hyper-immune sera.
This phenomenon might be considered an artifact,
as it is questionable if it will be encountered
in vitro
with natural immune
media.
2. The effect is logically explained simply by
overcrowding over time of the surface of
virions remaining to be neutralized with non-neutralizing antibodies being bound to
their antigenic determinants. This will in the
end create a blockage for the neutralizing
antibodies, thereby preventing the neutralization
of such virions.
3. Virions overcoated with non-neutralizing
antibodies remain infective, as long as they
have not been
included in a virus-antibody aggregate or caught by a neutralizing
antibody.
3.8. The formula for the regular in vitro antigen-antibody interactions.
The in vitro virus-antibody neutralization reaction proceeds as two elementary processes: 1) an ”over-neutralization” caused by virus aggregation by predominating non-neutralizing antibodies and 2) the regular and uncomplicated first-order reaction by binding of antibodies to antigenic neutralization determinants on the virions. Over-neutralization can be observed in a neutralization test only with antibody concentrations over a certain moderate level and will be negligible with reaction periods exceeding a certain limit.
For IgM antibodies, the reaction by neutralizing antibodies and the binding of non-neutralizing antibodies to their antigenic determinants were found to proceed as first-order reactions with extended reaction periods. The first-order process is therefore the fundamental antibody reaction in vitro, which can be utilized to the elaboration of tests of high sensitivity.
In the 1978 article [1], the following formula for this basic neutralization reaction, including also the antigen as a variable, was deduced from the relationships presented in Eqs. 3, 4, and 5 above:
(Eq.
6)
where
kst
is the standard neutralization rate factor, V0
is the virus titer (virus dose), T
is the reaction time, and D
is
the antibody titer, which in a neutralization test is the dilution
factor of that particular dilution of the antibody medium that
neutralizes the virus dose V0
to 1 TCID50
after a reaction period of T, and q
is the log antibody/log antigen equivalence factor of neutralization.
It will be understood that kst, the neutralization rate factor, is a characteristic expressing the neutralizing potency of an antibody medium. It is proportional to the antibody titer and dependent on the virus concentration to a degree determined by the factor q. It follows from the formula that the antibody titer obtained in a neutralization test and consequently the test sensitivity will be proportional to the length of the reaction period. The relationship between the antibody titer and reaction time is of first order, while the relationship between the virus titer or virus concentration and reaction time is exponential.
Temperature is an important variable, which is not shown directly in the formula but is included indirectly because the factor q's value is temperature-dependent. In the 1978 study, q was approximately 0.15 at 37 oC but 0.24 at 4 oC [1].
The
character and significance of Eq.
6 might
justify a more easily accessible version of the:antigen-antibody
interaction formula:
(Eq. 7) ,
where the symbols D and V0 are substituted with Ab and Ag (antibody and antigen titer). It
should be noted that while the virus titer and virus concentration
are identical terms, the antibody titer and antibody concentration
are not because of multiple antigenic determinants on a virion resulting in a variety of especially non-neutralizing antibodies.
Conclusions summarized.
1. The basic antigen-antibody interactions in a
neutralization test, where the aggregation is
eliminated, can
be expressed in a relatively simple formula comprising all variables.
2. The neutralization rate factor and the antibody
titer for an antibody medium, and
correspondingly the
sensitivity of the antibody test, are directly proportional to the
reaction
time.
3. A linear neutralization rate in a
virus-antibody mixture confirms that neutralization is
of first order, is non-reversible under
physiological conditions, and consequently will
follow the lines of the formula.
3.9 Implications of the regular lines of antigen-antibody interactions for the elaboration and performance of antigen-antibody tests.
Neutralization tests.
Neutralization of virus by antibody in a neutralization test is the combined effect of two separate reactions, i.e., 1) the regular first-order neutralization reaction, and 2) the over-neutralization that will be seen with moderate to high antibody concentrations and relatively short reaction times.
The level of sensitivity obtained in a neutralization test performed at 37 oC and with a reaction period of 24 hours will not be obtained in tests at 22 or 4 oC until after a reaction for 4 or 16 days, respectively, cf. Fig. 5. The reaction time should be long enough to ensure that over-neutralization will be negligible. Neutralization will then follow the lines of the regular, first-order reaction, implying that the sensitivity of the test will be strictly proportional to the reaction time. In the herpesvirus studies, an increase of the reaction time at 37 oC from 1 to 24 hours raised the sensitivity of a neutralization test by a factor of 16-18 and not by a factor of 24 because of a remaining aggregation reaction recorded after 1 hour of reaction.
A 37oC/24h neutralization test will be the ideal standard test. In 2008, the World Organization for Animal Health approved the 37oC/24h neutralization test as a reference standard test in controlling BoHV-1 infections [31].
ELISA modifications.
1. The conventional antibody ELISA
In a conventional antibody ELISA,
the antigen is coated on the wells of microtiter plates, and the
reaction to be visualized by the detecting reagent is the progressive
binding of antibodies to the antigens. In titrations, titers will
show the highest levels of the reacting antibodies to the antigenic
determinants fixed to the plates. Aggregation is excluded, so the
reaction between antigen and antibody in a dilution series of an
antibody sample will be of first order right from the start of the
reaction, involving that the sensitivity of an antibody ELISA will be
directly proportional to the reaction time.
For herpesvirus
37oC/24h test modifications, the conventional antibody
ELISA is 8 times more sensitive than the first-order neutralization
test.
The advantage of an
adjustable sensitivity can be illustrated by the following. In 1988,
the EU Commission issued a directive (88/406) committing member
countries to test their cattle herds twice at an interval from 4 to
12 months for the presence of infection with the bovine leukemia
virus, a retrovirus. Dairy herds could be tested for antibodies
on pooled milk samples twice with a specified interval, conditioned that not more than 20 cows were represented in a test sample. The
sensitivity requirements were indicated by a reference standard
sample distributed. In collaboration with G. Florent, Norden
Laboratories, the sensitivity of an antibody ELISA as described by
Portetelle et al. 1983 [37] was increased by changing the
reaction conditions to 37 oC for a long period, implying
that Danish dairy herds could be controlled on bulk tank milk
samples.
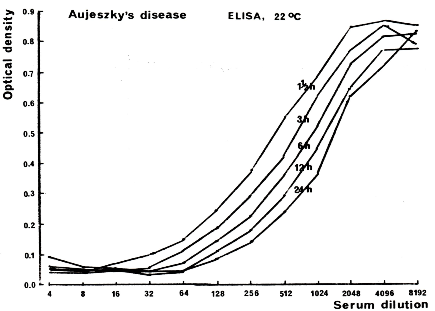
2. The blocking antibody ELISA
Sørensen and Lei 1986 [38] found that the reaction in a blocking antibody ELISA depended on both the reaction temperature and period. The reaction characteristics of a simple test version used with late-infection serum are illustrated in Fig. 9. A log-log linear relationship exists between the antibody titer and reaction time, but the reaction is not of first order but decelerating. An increase in the reaction time by a factor of 16 raised the antibody titer by a factor of approximately 4.
Figure 9. The kinetics of antigen-antibody binding in a serum dilution series as seen in a blocking ELISA for demonstration of SuHV1 antibodies. Bitsch, unpublished.
A twofold dilution series of a natural antibody-positive serum containing predominantly IgG antibody was allowed to react for 1.5, 3, 6, 12, and 24 hours. In the next steps, (1) a specific BoHV1 antibody preparation conjugated to biotin, (2) peroxidase conjugated to avidin, and (3) a suitable substrate were added. Steps 1, 2, and 3 were preceded by thorough washings. Negative samples will show an identical full-color development in the enzyme substrate, while positive samples will show full or partial blocking of color development. A titer can be recorded by the dilution factor of the dilution showing e.g. 50% of the optic density measured for negative samples. A linear relationship is seen to exist between the logarithmic values of antibody titer and reaction time, (decelerating rate).
The change in reaction from full blocking to no blocking occurs over an antibody titer interval of 5-6 log base-2 units. A log-log linear relationship exists between antibody titer and reaction time. An increase in the reaction time from 1 to 24 hours will raise the antibody titer by a factor of approximately 4 (decelerating rate).
In connection with the eradication of the three widespread respiratory viral SuHV-1, BoHV-1, and bovine viral diarrhea infections in Denmark after 1980, the blocking antibody ELISA was selected for routine testing of undiluted serum/plasma and bulk tank milk samples with reaction at 37 oC for close to 24 hours because of its simplicity and applicability for large-scale examinations.
For herpesvirus 37oC/24h test modifications, the sensitivity of the blocking ELISA was 2 times higher than that of the first-order neutralization test, although 4 times lower than that of the conventional antibody ELISA. This last-mentioned test was used for follow-up examinations or whenever a higher sensitivity was desirable.
3. The conventional antigen ELISA.
Antigen
ELISA versions, where specific capture antibodies are coated to the
wells of the microtiter plates, are widely used for diagnosing viral infections. The sensitivity will be determined by the frequency
of hits between capture antibodies and antigens, or actually the
lines of the antigen-antibody interaction formula, indicating an
exponential increase of the sensitivity by increasing reaction time.
From the data with extended reactions in Fig.
3, it
can be seen that 1) if for a constant concentration of the reacting
antibody the reaction time i increased by a factor of 2. the amount
of virus neutralized will be increased by approx. a factor of 100,
and 2) if for a constant reaction period, the concentration of the
reaction antibody is increased by a factor of 2, the concentration
of virus neutralized is increased by a factor of approx. 100. From
the early 1980s, routine examinations for various viral agents were
performed in the veterinary field in Denmark with antigen ELISA
versions with reaction at 37 oC for close to 24 hours. The test sensitivity was extremely high.
NB: Rapid and sensitive antigen and antibody ELISAs
Rapid and very sensitive antigen and antibody ELISAs can be
configured by including appropriate aggregation reactions. Principally, a conventional antibody ELISA is changed into a hybrid antibody ELISA by adding antigen to the wells of the ELISA plate after a relatively short reaction time, e.g., 30 minutes. This unfixed antigen reacts with the non-bound antibodies, whereafter the addition of complement will connect new antigen-antibody complexes in an antibody-positive sample to complexes already fixed to the plates. A hybrid antigen ELISA can be configured similarly by adding a monoclonal antibody to the wells after a short reaction time to capture unbound antigens in the test medium. whereafter addition of complement will result in rapid aggregation with fixed complexes.
Conclusions summarized.
1. The neutralization in a conventional
neutralization test proceeds as two separate reactions,
i.e., a first-order enduring reaction and an aggregation reaction
(”over-neutralization”) of
short duration.
2.
Highest sensitivity will always be obtained at a temperature not
below 37 oC.
3.
The conditions for an optimal neutralization test should be selected
accordingly:
over-neutralization should be negligible, the
reaction temperature should be 37 oC,
and the
reaction period should be extended appropriately.
4. A standard neutralization test for comparative
examinations, showing exclusively the reaction
with
neutralizing antibodies bound to their antigenic neutralization
determinant, will be
an assay with reaction at 37 oC
for 24 hours.
5. A conventional antibody ELISA is of first
order and the sensitivity is proportional to the
reaction
time.
6. The sensitivity of an antigen ELISA will be raised
exponentially with increased reaction
periods.
7. It will be possible to configure sensitive antigen and antibody hybrid ELISAs benefitting from the
rapidity of aggregation reactions
4. Concluding considerations regarding virus-antibody interactions.
The findings and conclusions from the analyses of antigen-antibody interactions in vitro in this review article are conflict with general concepts of antigen-antibody interactions in vitro, including also the relatively new so-called occupancy theory of neutralization (Parren and Burton 2001 [24], Burton et al.2001 [30]).
In Denmark, the control of the BoHV-1 infection was introduced in the artificial insemination bull centers in 1970 immediately after the eradication of the infection in these centers. A sufficiently high sensitivity of the antibody test used for control was crucial, so comprehensive analyses of the antigen-antibody interactions were conducted [1].
First, the reaction in a neutralization test was found to be bi-factorial, consisting of an early regular over-neutralization reaction and a neutralization reaction of first order. Second, virus-antibody bindings were found to be irreversible under physiological conditions. Third, the percentage law [3] was found invalid. Fourth, a particular temperature-dependent factor (q) was found to be an important co-determinator of the reaction rate and a central factor in virus-antibody interactions. Fifth, the relationships demonstrated made it possible to present the formula for the regular interactions, not comprising aggregation, between viruses and antibodies (Eqs. 6 and 7).
The implications were of the greatest importance. From the formula, it will be seen that the reaction in a constant-virus/varying-antibody test for demonstration of antibody will be of first order. A titer obtained, or the test sensitivity, will be proportional to the reaction time. As mentioned above, the increase in reaction time from 1 to 24 hours did not increase the sensitivity of a herpesvirus neutralization test by a factor of 24, but by a factor of 16-18 because of a residual over-neutralization by virus aggregation still being recorded after a reaction for 1 hour. Also, IgM antibodies, neutralizing and non-neutralizing, followed a first-order binding reaction with extended reaction times.
The huge neutralizing potency demonstrated for the non-neutralizing antibodies in a joint action with complement indicates a very important role of these antibodies in preventing and combating infectious diseases.
A
varying-virus/constant-serum test will be of no practical use for the
demonstration of antibodies because an antibody sample of relatively
low titer can easily neutralize virus in high concentrations, cf.
Fig.3.
Inversely,
however, for a test where a fixed quantity of antibody is used to
catch antigen from a test sample and where this reaction can be
visualized as in an antigen ELISA, the test sensitivity will be
raised exponentially
with
increased reaction time, because the value of the factor q is
considerably lower than 1, cf. Eq.
7.
It should be recalled that the purpose of the use of a test of high
sensitivity is not to obtain very high titers but to demonstrate a
reactant in low concentrations.
References
Bitsch V. An investigation into the basic virus-antibody neutralization reaction, with special regard to the reaction in the constant-virus/varying-serum neutralization test. Acta vet scand. 1978; 19:110-128.
Available from: http://viggobitscharticle1978a.blogspot.dk
Bitsch V, Eskildsen M. Complement-dependent neutralization of Aujeszky's disease virus by antibody. In: Aujeszky's Disease. Wittmann G, Hall SA, editors. Martinus Nijhoff Publishers, The Hague, Boston, London; 1982: 41-50.
Available from: http://bitschandeskildsenarticle1981.blogspot.dk
Andrewes CH, Elford WJ. Observations on anti-phage sera. I. "The percentage law". Brit J exp Path. 1933; 14: 307-376.
Burnet FM, Keogh EV, Lush D. Immunological reactions of the filterable viruses. Austr J exp Biol med Sci. 1937; 15: 231-368.
Dulbecco R, Vogt M, Strickland AGR. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956; 2: 162-205.
Reading SA, Dimmock NJ. Neutralization of animal virus infectivity by antibody. Arch Virol. 2007; 152: 1047-1059.
Klasse PJ. Neutralization of virus infectivity by antibodies: old problems in new perspectives. Advances in Biology. 2014. Article ID 157895.
Available from: http://dx.doi.org/10.1155/2014/157895Yoshino K, Taniguchi S. The appearance of complement-requiring neutralizing antibodies by immunization and infection with herpes simplex virus. Virology. 1964; 22: 193-201.
Yoshino K, Morishima T, Aoki Y. Requirement of neutralizing antibodies appearing after primary and booster immunization with herpes simplex virus. Jap J Microbiol. 1971; 15: 53-62.
Oldstone MBA. Virus neutralization and virus-induced immune complex disease. Prog med Virol. 1975; 19: 84-119.
Cooper NR, Nemerow GR. Complement-dependent mechanisms of virus neutralization. In: Immunobiology of the complement system. Ross GD. editor. 139-162. Academic Press, Harcourt Brace Janovich, Publishers. 1986.
Goldsby RA, Kindt TJ, Osborne BA. Kuby Immunology 4th ed. W.H. Freeman and Company; New York; 2000.
Bitsch V, Eskildsen M. A comparative examination of swine sera for antibody to Aujeszky's disease virus with a conventional and a modified virus-serum neutralization test and a modified direct complement fixation test. Acta vet scand. 1976; 17: 142-152.
Brioen PD, Dekegel, Boeyé A. Neutralization of poliovirus by antibody-mediated polymerization. Virology. 1983; 127: 463-468.
Thomas A.A.M, Brioen P, Boeyé A. A monoclonal antibody that neutralizes poliovirus by cross-linking virions. J Virol. 1985; 54: 7-13.
Thomas, A.A.M, Vrijsen R, Boeyé A. Relation between poliovirus neutralization and aggregation. J Virol. 1986; 59: 479-485.
Klasse PJ, Sattentau QJ. Mechanisms of virus neutralization by antibody. Current Topics in Microbiology and Immunobiology. 2001; 260: 87-108.
Bitsch V. The IBR/IPV virus-serum neutralization test. Studies on the influence of the virus-serum incubation prior to inoculation. Acta vet. scand. 1973; 14: 767-769.
Bitsch V. The P37/24 modification of the infectious bovine rhinotracheitis virus-serum neutralization test. Acta vet scand. 1978; 19: 497-505.
Ashe WK, Notkins AL. Neutralization of an infectious herpes simplex virus-antibody complex by antiglobulin. Proc Nat Acad Sci. 1966; 56: 447-451.
Male D, Brostoff J, Roth DB, Roitt IM. Immunology. 8th edition. Elsevier; 2012
Murphy K. Janeway's immunobiology. 8th edition. Garland Science; London, New York; 2012.
Svehag S.-E. Formation and dissociation of virus-antibody complexes with special reference to the neutralization process. Prog med Virology. 1968; 10: 1-63.
Parren, PWHI, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Advances in Immunology. 2001; 77: 195-262.
Mandel B. Reversibility of the reaction between poliovirus and neutralizing antibody of rabbit origin. Virology. 1961; 14: 316-328.
Jerne NK, Avegno P. The development of the phage-inactivating properties of serum during the course of specific immunization of an animal: reversible and irreversible inactivation. J Immunol. 1956; 76: 200-208.
Andrewes CH, Elford WJ. Observations on anti-phage sera. II. Properties of incompletely neutralized phage. Brit J exp Path. 1933; 14: 376- 383.
Massey RJ, Schochetman G. Viral epitopes and monoclonal antibodies: isolation of blocking antibodies that inhibits virus neutralization. Science. 1981; 213: 447-449.
Icenogle J, Schiwen H, Duke G, Gilbert S, Rueckert SR, Anderegg J. Neutralization of poliovirus by a monoclonal antibody: kinetics and stoichiometry. Virology. 1983; 127: 412-425.
Burton DR, Saphire EO, Parren PWHI. A model for neutralization of viruses based on antibody coating of the virion surface. Current Topics in Microbiology and Immunology. 2001; 260: 109-143.
OIE recommendation. Terrestrial Manual, Chapter 3.1.2.
Gard S. Neutralization of Theiler's virus. Acta path microbiol scand. 1955; 37: 21-30.
Gard S. Immuno-inactivation of poliovirus. Arch ges Virusforsch. 1957; 7: 449-460.
Svehag S-E. Effect of different contact conditions on the blue tongue virus-antibody reaction and on the validity of the "percentage law". Arch ges Virusforsch. 1963; 12: 678-693.
Svehag S-E, Mandel B. The formation and properties of poliovirus-neutralizing antibody. J exp Med. 1964;119: 1-19.
Kramps JA, Quak S, Weerdmeester K, van Oirschot JT. Comparative study on sixteen enzyme-linked immunosorbent assays for the detection of antibodies to Bovine herpesvirus 1 in cattle. Vet Microbiol. 1993; 35: 11-21.
Portetelle D, Bruck C, Mammerickx M, Burny A. Use of monoclonal antibody in an ELISA for the detection of antibodies to Bovine leukemia virus. J virol Methods. 1983; 6: 19-29.
Sørensen KJ, Lei JC. Aujeszky's Disease: blocking ELISA for the detection of serum
antibodies. J virol Methods. 1986; 17: 171-181.